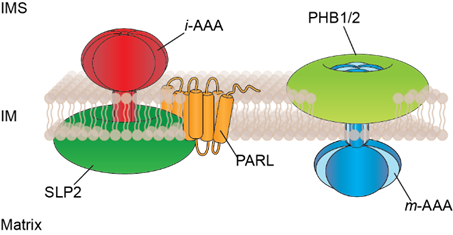
P01 - The role of membrane scaffolds for the spatial organization and heterogeneity of the mitochondrial inner membrane
The mitochondrial inner membrane is a protein-rich, complex membrane structure, which serves as an important metabolic barrier in cells and houses numerous metabolic processes. Distinct functions are carried out in morphologically defined regions, such as protein translocation in the inner boundary membrane or the production of ATP via OXPHOS complexes in mitochondrial cristae. Moreover, increasing evidence suggests large functional heterogeneity at the nanoscale within the inner membrane. Multimeric membrane scaffold proteins of the SPFH-family, prohibitins (PHB1, PHB2) and the stomatin-like protein SLP2, form large ring complexes in the inner membrane, which are proposed to serve as lipid and protein scaffolds. These scaffold proteins assemble with AAA proteases at opposite membrane surfaces constituting proteolytic hubs in the inner membrane. Loss of PHB membrane domains severely impairs mitochondrial functions and is associated with neurodegeneration, obesity and kidney failure.
During the last funding period, we have identified TMBIM5 (GHITM, MICS1) as a novel constituent of PHB membrane domains. Our functional analysis revealed that TMBIM5 acts both as a Ca2+/H+ exchanger and inhibitor of the m-AAA protease in the inner membrane. TMBIM5 thus couples the mitochondrial proton gradient with protein turnover rates to reshape the mitochondrial proteome and adjust the cellular metabolism. These findings suggest that spatially restricted, localized proteolysis contributes to the functional and structural heterogeneity of the inner membrane. We will therefore examine the role of membrane scaffolds and the TMBIM5-dependent proton gradient for the spatial regulation of proteolysis in the inner membrane. We will apply various advanced proteomic approaches to monitor localized proteolysis at PHB membrane domains, define the protein topology within these domains and examine the effect of TMBIM5 and the proton motif force on the stability of inner membrane proteins. Together, our experiments will further define regulatory circuits coupling protein turnover to the energetic status of mitochondria and unravel how localized proteolysis regulated by membrane scaffolds contributes to the functional heterogeneity of the inner membrane.