P02 - Crosstalk and metabolic control of mitochondrial inner membrane-shaping protein machineries
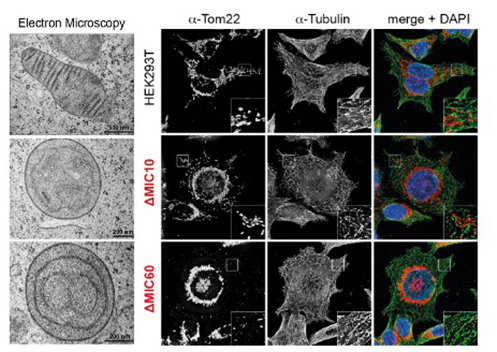
The native architecture of mitochondrial cristae is central for optimal respiration and mitochondrial physiology. The mitochondrial contact site and cristae organizing system (MICOS) and higher order assemblies of the F1Fo-ATP synthase both perform important, but distinct functions in generating cristae architecture. However, it is so far unknown if and how these machineries coordinate their complementary activities in cristae biogenesis. We have recently shown that the MICOS core subunit Mic10 interacts with ATP synthase dimers and regulates their association into oligomeric rows using yeast as a model system. This unexpected role of Mic10 is crucial for mitochondrial physiology and cellular adaptation to growth conditions that require robust respiratory metabolism. These novel insights into the (re-)organization of membrane-shaping protein machineries allow us now to perform precisely tailored studies on the underlying regulatory processes in space and time. Employing a plethora of biochemical, genetic and super-resolution imaging approaches, we will dissect how MICOS and the F1Fo-ATP synthase individually and in coordination govern inner membrane remodeling during metabolic adaptation and related cristae biogenesis regimes. Building on our previous work, we will employ a genetically engineered fusion protein that allows for triggered release of caged Mic10. With this tool, we will be able to monitor Mic10 oligomerization, MICOS assembly and eventually crista junction formation with high spatial and temporal resolution. These studies will be complemented by a detailed biochemical analysis of Mic10 oligomer architecture and the mechanism of Mic10's dual functions in membrane shaping. In line with recent evidence that Mic10 functions at both MICOS and F1Fo-ATP synthase are evolutionarily conserved, our preliminary data indicate that a fraction of human Mic10 is bound to the ATP synthase. Therefore, we will study the molecular crosstalk of Mic10 and IF1, a known regulator of ATP synthase oligomerization, in a human cell model. Taken together, our work will provide important novel insights into regulatory mechanisms that govern the rosstalk of mitochondrial membrane-shaping machineries and open up new perspectives on the metabolic remodeling of mitochondria.