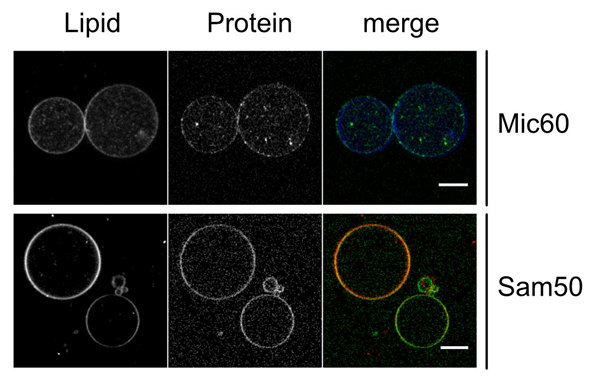
P05 - Molecular characterization of protein-dependent inner mitochondrial membrane morphogenesis
The mitochondrial inner membrane (IM) displays a complex, convoluted architecture with regions of high degrees of membrane curvature. Several proteins and lipid species have been attributed a crucial function in the formation and stabilization of this ultra-structure. Important morphology defining elements of the inner mitochondrial membrane are crista junctions (CJs). This short, tubular membrane segments displays high degrees of membrane curvature and connect the inner boundary membrane with cristae membranes. The multi-subunit mitochondrial contact site and cristae organizing system (MICOS) is required to maintain and most probably form CJs. Other IM shaping molecules include the FOF1-ATP synthase, the optic atrophy 1 (Opa1) as well as the non-bilayer lipids phosphatidylethanolamine (PE) and cardiolipin (CL). Both, CL and PE, are synthesized in the IM from precursors that are transported by lipid transfer proteins from the OM to the IM. Interestingly, direct and indirect connections between these structure relevant molecules, in form of protein-protein but also protein-membrane interactions, have been described, though the molecular details of most of these interactions are yet to be discovered.
In this project, we aim to characterize these interactions and how they affect the morphology and biogenesis of the IM. The MICOS complex consists of proteins with the ability to remodel membranes and to form extensive protein-protein interaction networks. While some progress has been made in assigning specific functions to single MICOS subunits, a detailed understanding of how the various complex proteins act in a concerted manner, but also with other proteins and lipids, is largely missing. The MICOS core subunit Mic60 displays dual function. On the one hand, Mic60 actively remodels membranes at CJs and, on the other hand, it provides multiple protein-protein interactions, some of which crucial for OM-IM contact sites. If and how these two functions are coupled is poorly understood and will be addressed in this project. As OM-IM contact sites might also provide regions for enhanced lipid transfer between these two membranes, we will include lipid transfer proteins in our experiments. Based on our results showing a membrane morphology dependent activity of these proteins, we aim to investigate how lipid transfer works in the presence of MICOS-mediated membrane contact sites, Mic60-induced membrane curvature and other IM scaffolding proteins.